
Interfacing materials. Interfacing disciplines.
Home | PI Info | Research | Publications | Mentoring/Outreach | Links
Atomic Layer Deposition (ALD) to Stabilize Molecular Dyes in Photoelectrochemical Systems for Solar Fuels
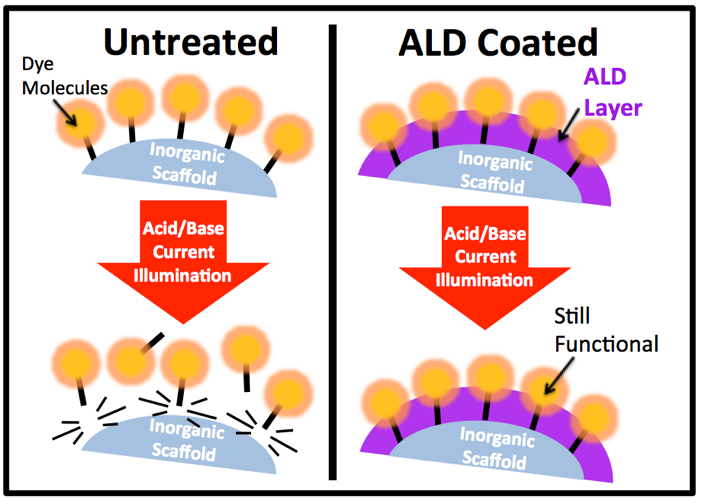
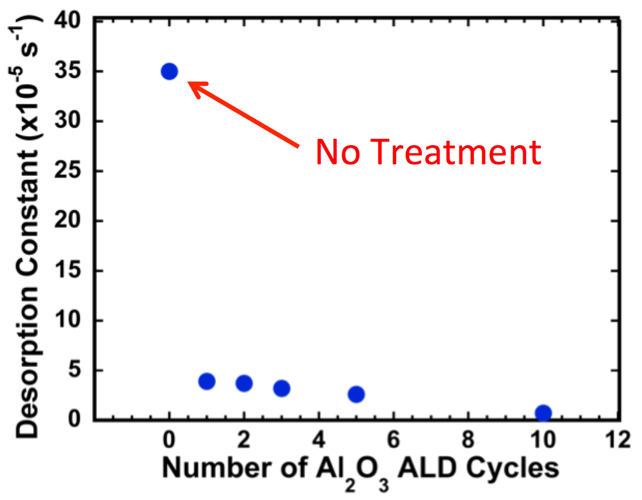
Fig. 1: Illustration of how ALD can be used to protect surface attached molecules. Depiction on the left shows how the addition of a sub-nanometer ALD layer can improve the attachment durability of molecular dyes even under chemical attack, electrical current flow, or illumination. Data on the right shows the order of magnitude decrease in desorption rate for ALD protected dye molecules attached to mesoporous TiO2.
Related Publications
1. M. D. Losego, K. Hanson, “Stabilizing molecular sensitizers in aqueous environs.” Nano Energy. 2 1067 (2013). DOI
2. K. Hanson, M. D. Losego, B. Kalanyan, G. N. Parsons, and T. J. Meyer, “Stabilizing small molecules on metal oxide surfaces using atomic layer deposition.” Nano. Lett. 13 4802 (2013). DOI
-
3.K. Hanson, M. D. Losego, B. Kalanyan, H. Luo, D. L. Ashford, G. N. Parsons, and T. J. Meyer, “Stabilization of [Ru(Bpy)2(4,4’-(PO3H2)bpy)]2+ on TiO2 with Atomic Layer Deposition of Al2O3” Chem. Mater. 25 3 (2013). DOI
-
4.A. K. Vannucci, L. Alibabaei, M. D. Losego, J. J. Concepcion, B. Kalanyan, G. N. Parsons, T. J. Meyer, “Crossing the divide between homogeneous and heterogeneous catalysis in water oxidation.” Published Online: Proc. Nat. Acad. Sci. (2013). DOI
The small-scale commercialization of dye-sensitized solar cells is now being realized because the challenges to long-term stability have been overcome. However, using molecular modifiers, like metal-organic dyes and catalysts, in aqueous environments poses an even greater challenge because anchor group chemistries are susceptible to hydrolysis and desorption. Illumination, pH, and electrical current flow necessary for photoelectrochemical device operation further enhance rates of molecular detachment.
Working with colleagues at the UNC EFRC for Solar Fuels and the Research Triangle Solar Fuels Institute, we are developing strategies using atomic layer deposition to dramatically improve attachment of these molecular modifiers. Unlike prior work, we are applying < 1 nm coating via ALD after attachment of molecular modifiers to mesoporous electrode scaffolds. In our initial publication [3] we demonstrate over an order of magnitude decrease in desorption rates for ALD protected dye molecules on a mesoporous TiO2 photoelectrode under illumination (see Fig. 1) and at much higher pH values than unprotected photoelectrodes. Research is ongoing to better understand the underlying chemical modifications during ALD treatments and the implications for electron transfer kinetics.