RESEARCH
Crystallization of ALD Thin Films
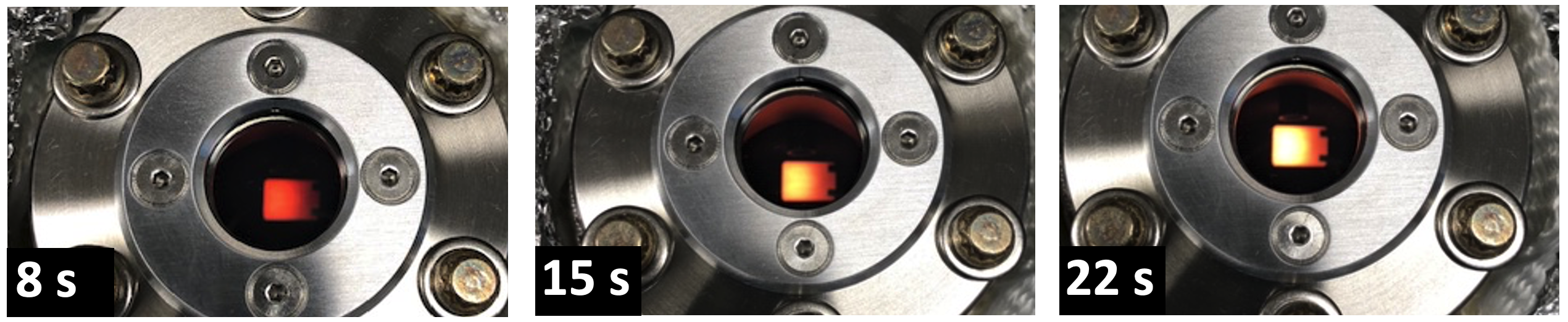
We have interest in the fundamental kinetics of ALD film crystallizations. For example, we have shown that TiO2 crystallization from the TDMAT / water chemistry is rate limited by the nucleation process, with crystal growth having a near-zero activation barrier. By identifying sub-oxidation of TiO2 being the chemical barrier to nucleation for thermal ALD, we were able to introduce an additional oxidation step to lower the nucleation barrier and achieve the lowest temperature and lowest thickness crystallization behavior for this chemistry via a thermal ALD process. We are also interested in creative ways to drive crystallization, including the application of pulsed heating between ALD dosing steps to independently control film crystallization (or epitaxial growth) using precursor that otherwise have low decomposition temperatures.
Structure and Hydration Behavior of Amorphous ALD Materials
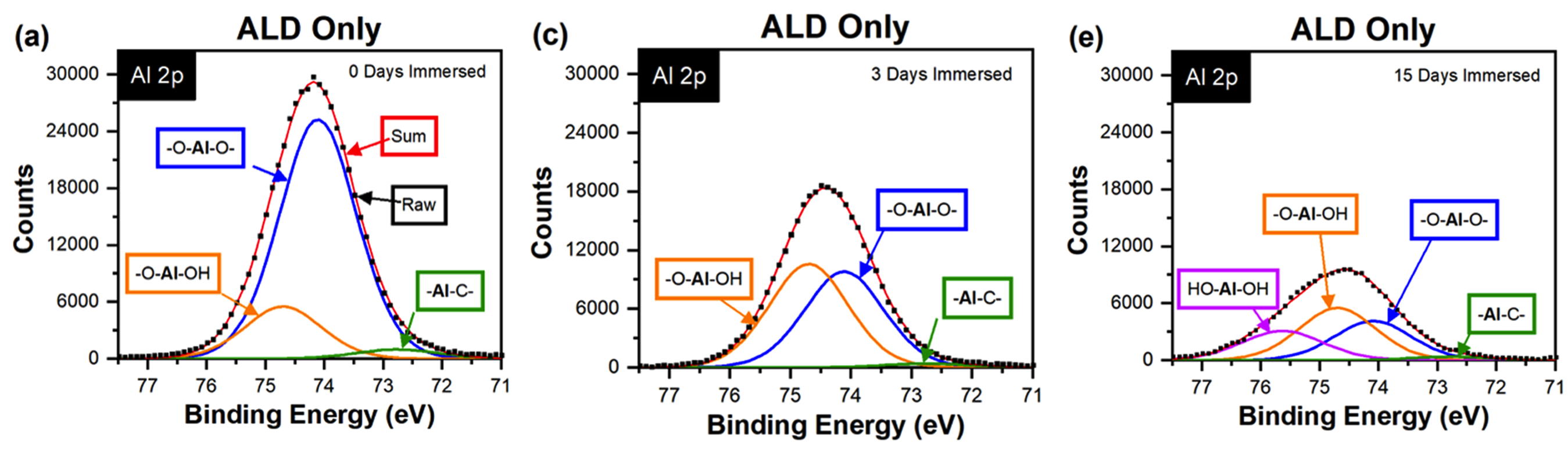
While many ALD materials deposited at low temperature are amorphous, different growth temperatures can alter the amorphous structure, even when within the ALD temperature growth window. These differences in structure lead to difference in properties including refractive index and thermal conductivity. Related, these materials can have different hydration states (hydroxides) and the interactions with water and how these materials hydrate or even dissolve have important in a variety of applications.
ALD Coatings of Powders
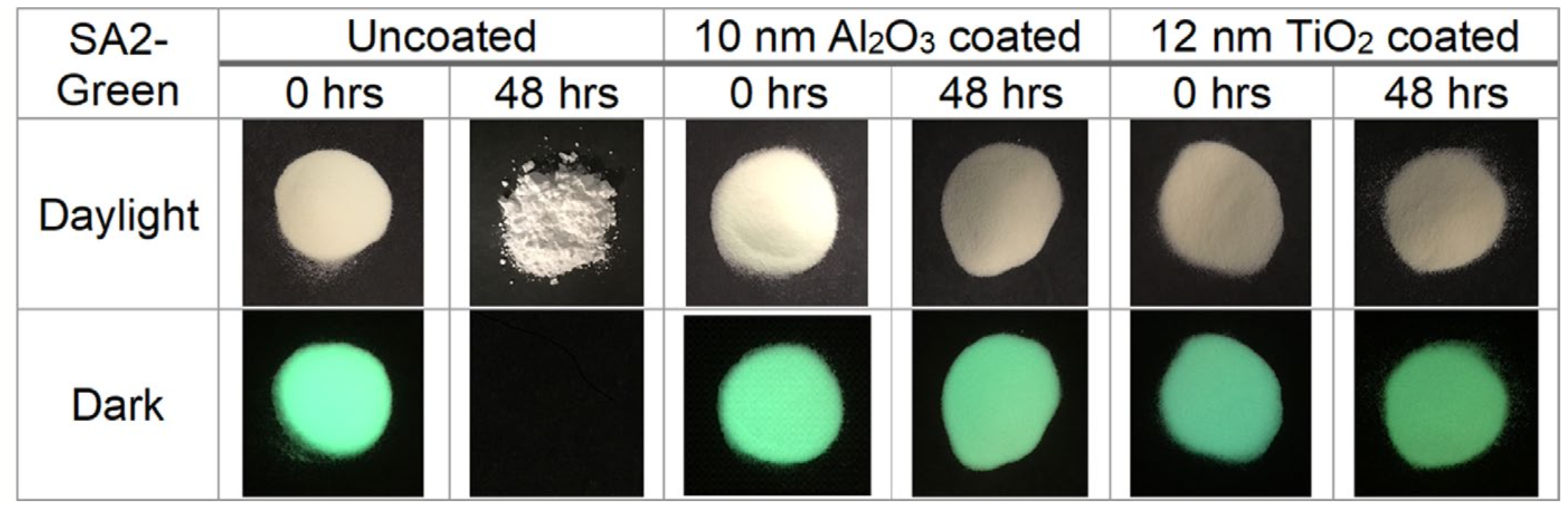
Many modern products are formed from powders (batteries, sintered ceramics, 3D printed metals and ceramics, composites, etc.). Chemical modifications to the surfaces can alter various properties. We have used ALD to “glue” molecular catalysts to surfaces to create more easily recyclable heterogeneous catalysts with the specificity of molecular homogeneous catalysts, and have protected water-sensitive long-afterglow (>12 h) phosphors from environmental degradation.
ALD Coatings of Cellulosic Products and other Commodity Goods

We have interest in how ALD can be apply to commodity goods, particularly when a single ALD cycle or a “few” ALD cycles can significantly alter the properties of the material. One example is how a single or double ALD cycle of various metal oxides can transform various cellulosic products (including cotton balls, cotton fabrics, nanocellulose, and wood lumber) into hydrophobic materials. We have shown how this is a combination of both surface chemistry and surface structure. In one example, we have transformed fibrous cotton mats into oil-selective sorbents for marine-based oil spill remediation, in another, we have shown how single-cycle ALD can make lumber resistant to water uptake, warpage, and mold growth.